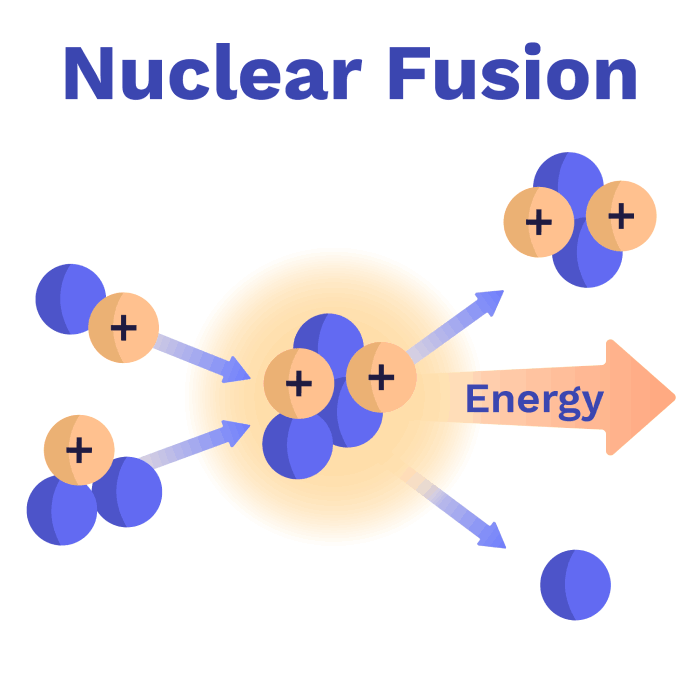
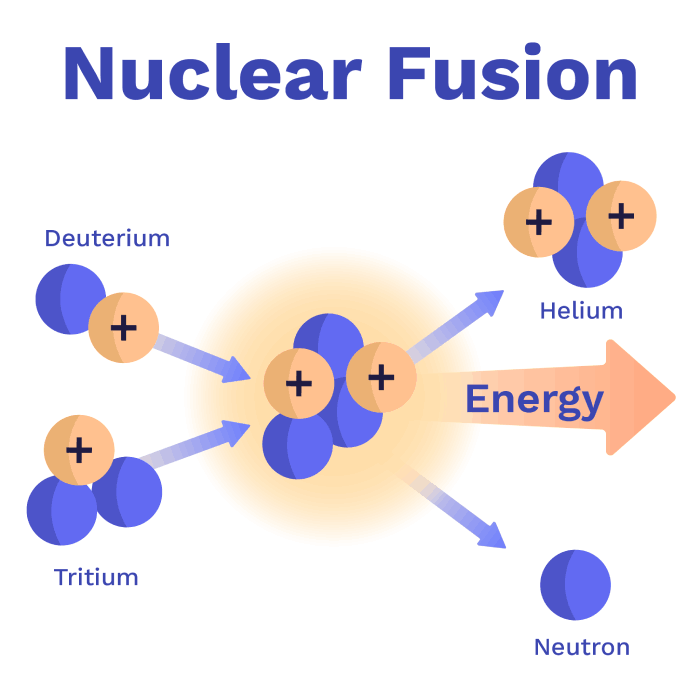
Luckily, helium is not dangerous - it’s what’s used to fill balloons. Now that we know the elements involved, let’s look at the reaction again:
Why does this release energy?
As weird as it sounds, Helium plus one neutron has less mass than Deuterium plus Tritium . Yes, really! By recombining the same number of protons and neutrons into a different configuration, the whole nucleus loses mass
. Yes, really! By recombining the same number of protons and neutrons into a different configuration, the whole nucleus loses mass .
.
The lost mass is converted to energy, which is released in the form of heat and electromagnetic radiation. Einstein’s famous equation E = mc² is at work here : we are converting mass into energy.
: we are converting mass into energy.
So why aren’t we using fusion to make energy?
In the sun, fusion occurs because of the intense pressure in the Sun’s core caused by gravity. It gets as hot as 15,000,000°C !
!
The good news is we can create fusion on Earth. The bad news: it’s really difficult.
To date, all fusion reactors use more energy than they produce
 . This is, of course, a problem. A power plant that uses more power than it produces is useless.
. This is, of course, a problem. A power plant that uses more power than it produces is useless.
The ratio of input to output energy is often called “Q”. Nuclear fusion has a long history, but, so far, we haven’t managed to get Q to 1

 :
:
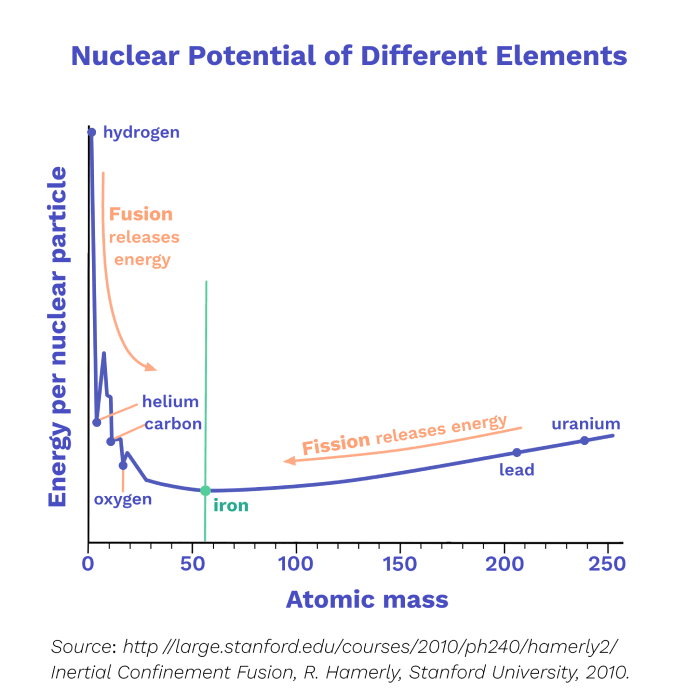
As you can see from this graph, we got so close to Q=1, but then improvements stopped happening - why? In the next chapter, we’ll explore what the graph above means, discover what prevented further progress (so far), and discuss recent work that aims to get to Q=10 and higher.
Next Chapter? Could we use the same mechanism to produce energy here on Earth? The answer to both these questions is nuclear fusion
!

.